Understanding the Basics of PICOT Questions
What is the PICOT Framework?
- The PICOT framework is a structure used to formulate PICOT questions in evidence-based practice, particularly in healthcare and nursing.
- P stands for Patient or population, identifying the group you are studying.
- I refers to the Intervention or exposure that you are interested in exploring.
- C is for Comparison, where you compare different interventions, treatments, or approaches.
- O stands for Outcome, the effect or result you are measuring.
- T is the Time frame, defining how long the study lasts.
Get Expert Writing Help
Need expert assistance with your dissertation or systematic literature review? Get professional writing support from Best Dissertation Writers and ensure your research is comprehensive, well-written, and meets academic standards. Contact us today!
The Importance of PICOT Questions in Evidence-Based Practice
- PICOT questions are essential in evidence-based practice as they provide clarity and focus when researching a clinical issue.
- These questions help in formulating research that leads to actionable results in healthcare settings.
- They guide researchers to find evidence from systematic reviews, randomized controlled trials, and other high-quality research articles available in libraries such as PubMed or medical journals.
- By clearly defining the components (e.g., P, I, C, O, and T), PICOT questions help ensure the literature review is specific and relevant to the diagnosis, treatment, or disease being studied.
How to Identify the Components of a PICOT Question
- To develop effective PICOT questions, start by identifying the patient population, which could be an adult group in a clinic or hospital setting.
- Next, specify the intervention, which could be a particular treatment or medication.
- Identify a comparison group, which could be a standard diagnosis or healthcare procedure.
- Define the outcome you are studying, such as recovery rate, disease progression, or patient satisfaction.
- Finally, establish the timeframe of the study, whether it is a short-term treatment or long-term medicine management.
Using the PICOT format, one can create focused research questions to guide clinical research and education in the field of medicine and healthcare. This structured approach allows practitioners and researchers to review relevant articles and literature, ensuring that they find the most appropriate and effective solutions for their studies.
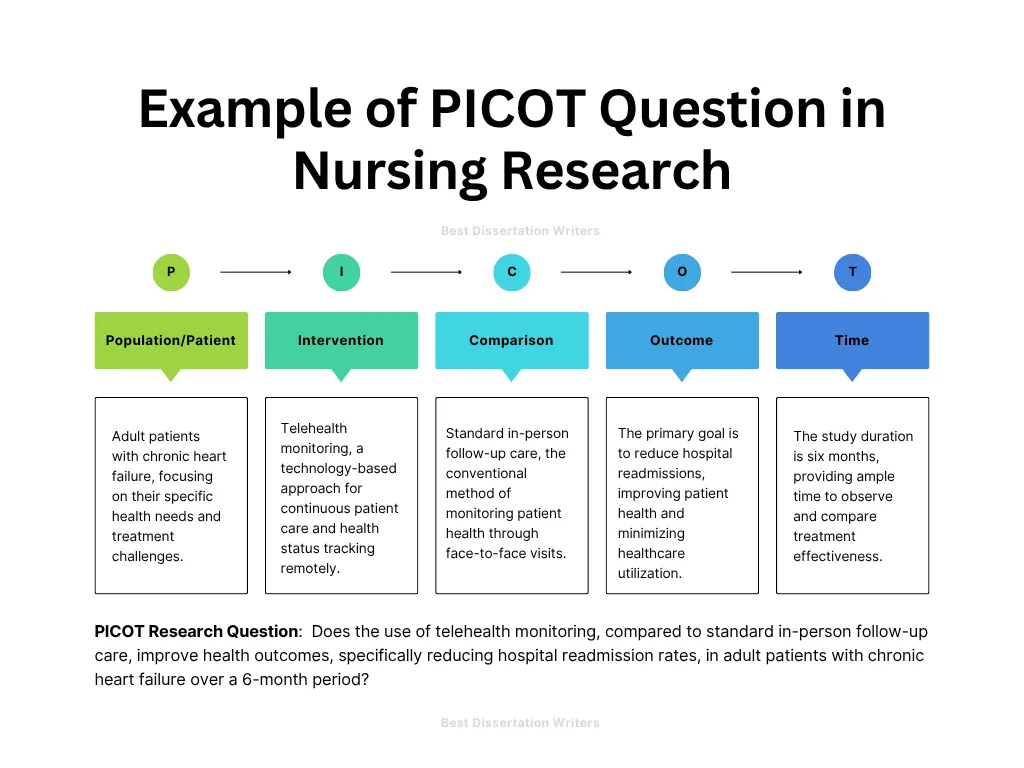
Here is an example of PICOT Research Question:
Does the use of telehealth monitoring, compared to standard in-person follow-up care, improve health outcomes, specifically reducing hospital readmission rates, in adult patients with chronic heart failure over a 6-month period?
| Variable | Definition |
| P (Population) | Adult patients with chronic heart failure (CHF) |
| I (Intervention) | Telehealth monitoring |
| C (Comparison) | Standard in-person follow-up care |
| O (Outcome) | Health outcomes, specifically reducing hospital readmission rates |
| T (Time) | Over a 6-month period |
5 Step-by-Step Guide to Developing Effective PICOT Questions
Identifying Your Clinical Question and Study Population
- The first step in developing PICOT questions is to clearly define the clinical question that you are researching. This helps to focus the research and guides the study design.
- The study population should be specific to the patients with chronic conditions, or any other group related to your clinical question. Consider characteristics such as age, gender, and health status.
- Think about the epidemiology of your study population, as it helps identify the variables that could impact your research.
- You can find relevant data in systematic reviews and meta-analysis, which help you find the best evidence for your study.
- Use the library at the university or online research guides to gather additional literature for a well-defined study population.
Selecting the Intervention and Comparison for Your Research
- In PICOT questions, you need to specify the intervention and the comparison. The intervention refers to the specific treatment, procedure, or therapy being studied.
- The comparison could be the usual care or a different treatment approach. This step is critical to understand how the new intervention fares against an established one.
- For example, in a study examining recovery time in patients with chronic illnesses, the intervention could be a new med regimen, and the comparison might be the usual care.
- This section should be clear so that you can measure the reduction or improvement in the outcome based on the two interventions.
Defining Outcomes and Time Frame for Evidence-Based Research
- Defining the outcomes is key to measuring the success or failure of an intervention in PICOT questions. Outcomes should be measurable and meaningful in the context of your research.
- Examples of outcomes include patient care improvement, screening results, or recovery time after treatment. The outcome must be aligned with the research question.
- Additionally, establish the time frame (T) for the study. Time frames in health sciences research can range from days to years, depending on the disease or intervention.
- Make sure to define the duration for which you will measure the outcome. For example, you may look at the recovery time for patients with chronic disease at 3 months or 6 months after treatment.
Clarifying the Comparison Group or Control
- The comparison group (C) or control is critical in understanding the effect of the intervention.
- Ensure that the comparison group is similar to the study population in characteristics, but does not receive the intervention.
- The comparison group could include patients with chronic conditions who receive usual care or a different treatment. This allows you to compare the reduction in symptoms or other outcomes.
- You can gather this information through research guides, such as those available in the library at the university, or from data on epidemiology and previous studies in the same field.
Refining the Time Frame (T)
- Refining the time frame (T) in your PICOT questions is crucial as it directly impacts the measurement of the outcome.
- Decide how long it will take for the intervention to show a meaningful effect. For instance, if you are studying a treatment for patients with chronic conditions, the time frame might be a year for long-term outcomes.
- Consider how the time frame will impact patient care and outcomes. Some outcomes may need more time to manifest, while others may show quicker results.
- Use systematic reviews and meta-analysis to guide your decision on a time frame that aligns with industry standards and research findings.
By following these steps, you will have a clear framework for formulating effective PICOT questions, ensuring that your research is structured and focused on addressing key issues in healthcare.
Leveraging Resources and Databases for Effective PICOT Question Development
Using Clinical Databases for Searching PICOT Questions
- Clinical databases like PubMed, CINAHL, and Cochrane Library are invaluable tools when developing PICOT questions.
- These databases allow you to access a wide range of primary research articles, systematic reviews, and clinical studies that can inform your PICOT questions.
- PICOT questions are grounded in evidence, and databases provide you with expertise and peer-reviewed materials to refine your question based on the latest research findings.
- Searching these databases will help you find studies that are directly related to your participant population, ensuring your research question is both relevant and focused.
Reference Tools to Enhance PICOT Question Creation
- Using reference tools like EndNote, Zotero, and Mendeley can streamline the development of PICOT questions by organizing and managing your citations.
- These tools help ensure that the references you use in developing PICOT questions are reliable and well-cited, enhancing the credibility of your work.
- With these tools, you can efficiently track the keywords used in your PICOT questions, such as intervention, population, and outcome, improving your search process for high-quality evidence.
Exploring Levels of Evidence in Database Searches
- When developing PICOT questions, it’s essential to understand the levels of evidence that exist within the database search results.
- Research studies are categorized based on their strength, from expert opinions and case reports to primary randomized controlled trials (RCTs) and meta-analysis.
- By focusing on higher levels of evidence, such as systematic reviews and meta-analysis, you ensure that your PICOT questions are grounded in robust evidence, increasing the validity of your research outcomes.
- Exploring these levels helps you to formulate PICOT questions that are more likely to contribute to improved patient care and clinical decision-making, based on evidence-based practices.
Leveraging these resources and tools enhances your ability to develop well-structured PICOT questions with strong evidence to back them, improving the quality and relevance of your research.
Tips and Strategies for Refining Your PICOT Questions
Crafting Clear and Focused PICOT Questions
- When developing PICOT questions, clarity is key. Make sure the question is focused on a specific participant population, intervention, comparison, and outcome.
- A well-crafted PICOT question ensures that the research is precise, which improves the overall quality and relevance of the study.
- Avoid vague terms and focus on measurable variables that will lead to clear answers. For example, instead of asking, “Does treatment help?”, ask, “What is the impact of a new drug on recovery time in patients with chronic conditions?”
- A focused PICOT question helps narrow down the search results and guides you to the most relevant studies.
Evaluating the Relevance of Your PICOT Question to Research Goals
- Before finalizing your PICOT question, evaluate its relevance to your research goals. Ensure that the question aligns with the specific problem you intend to address.
- Ask yourself if the question will lead to answers that are meaningful for clinical practice. For instance, will the PICOT question improve patient care or contribute to better treatment options?
- Relevance is essential when searching for evidence; the more aligned your PICOT question is with your research goals, the more efficiently you can find high-quality sources.
Advanced Search Strategies for Finding the Best Resources
- PICOT questions are best supported by high-quality evidence. Utilize advanced search strategies in databases like PubMed, CINAHL, or Cochrane Library to find primary research articles and systematic reviews.
- Use Boolean operators (AND, OR, NOT) and filters to narrow down your search results to studies that meet the PICOT question criteria. For example, include keywords such as “chronic disease,” “treatment,” and “outcome” to find studies relevant to your population and intervention.
- The expertise found in systematic reviews or meta-analyses can guide the refinement of your PICOT questions, ensuring you are using the most current and evidence-based information.
By following these strategies, you can develop well-defined PICOT questions that will guide your research process and help in finding the best evidence to address critical healthcare issues.
