What Is Inclusion and Exclusion Criteria in a Systematic Review?
Defining Inclusion and Exclusion Criteria as Eligibility Criteria
- Inclusion and exclusion criteria are eligibility criteria that clearly specify which studies or study participants are eligible for inclusion and which must be excluded.
- In a systematic review, these criteria are used to select articles.
- In clinical research or a clinical trial, they are used to determine who can participate in the study.
- Eligibility criteria are established based on the research question.
- The research question guides what type of population, intervention, comparison, and outcomes are relevant.
- The criteria that will be used must align directly with the objectives of the review based on your research question.
- Inclusion criteria define the appropriate inclusion of studies or individuals.
- Inclusion criteria may specify demographic factors such as age, sex, or geographic location.
- They may define medical conditions such as heart failure or other chronic conditions.
- They may specify type and stage of disease, previous treatment history, or type of intervention.
- Exclusion criteria are defined to remove irrelevant or unsuitable studies or participants.
- Exclusion criteria must clearly state which characteristic leads to exclusion.
- For example, studies involving pregnant women may be excluded if the focus is a different target population.
- Individuals with severe comorbidity or additional medical conditions may be excluded to reduce confounding factors.
- In a literature review, inclusion in a literature review depends on meeting predefined standards.
- The research team may review inclusion and exclusion criteria during screening.
- These criteria help retrieve and select articles for your literature review in a systematic and unbiased way.
- Establishing inclusion and exclusion criteria is crucial to the success of the study.
- Without appropriate inclusion and exclusion criteria, the review or trial may lack clarity.
- Poorly defined eligibility criteria can affect the study results and reduce credibility.
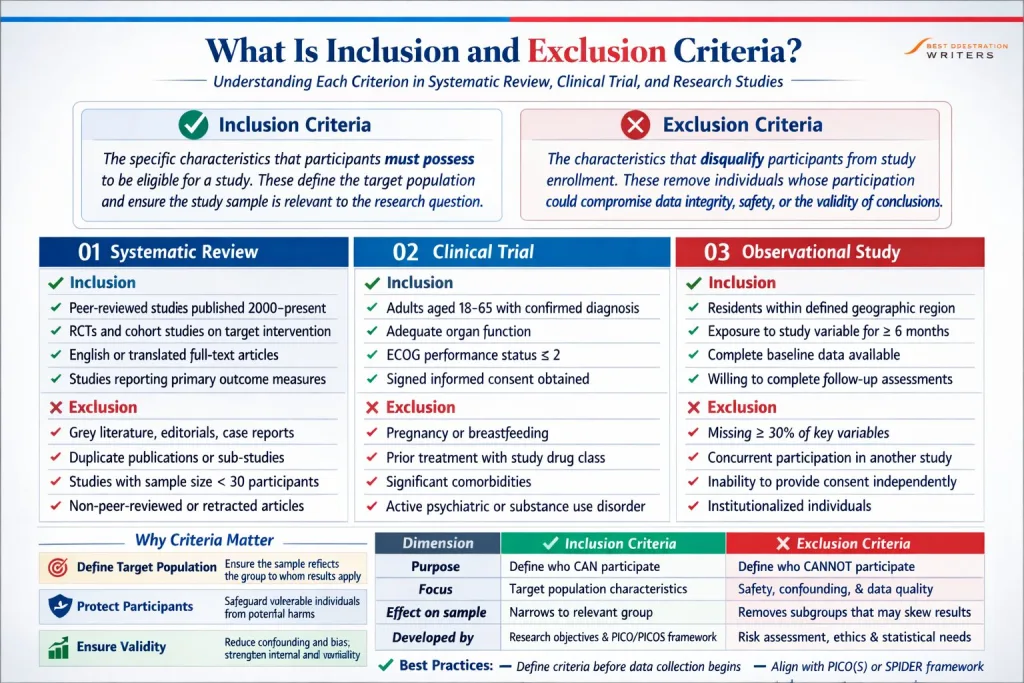
Understanding Each Criterion and Its Role in Study Selection
- Each criterion represents a specific characteristic that must be assessed during screening.
- In systematic reviews, researchers screen titles, abstracts, and full-text articles.
- Each potential article is evaluated against the criteria that will be used.
- Criteria are used to identify relevant studies.
- For example, study design may be limited to randomized controlled trials.
- The intervention must match the topic under investigation.
- The population must reflect the defined target population.
- Screening involves structured steps.
- Initial screening of abstracts to exclude clearly irrelevant studies.
- Full-text screening to verify eligibility.
- Exclusion criteria with each potential article are documented for transparency.
- In clinical trials, eligibility criteria determine who can join.
- Study participants must meet the inclusion criteria before joining a clinical trial.
- Exclusion criteria must be reviewed carefully before recruitment.
- Participants must provide informed consent before they participate in the study.
- Examples of common eligibility factors include:
- Age range and demographic characteristics.
- Type and stage of illness.
- Previous treatment history.
- Ability to attend study visits and follow-up study appointments.
- Willingness to complete a questionnaire or comply with study procedures.
- Eligibility criteria help ensure that participants are representative of the target population.
- When participants are representative, results of the study are more generalizable.
- This improves external validity.
- In systematic reviews, criteria are used to select articles and ensure consistency.
- Articles for your literature review must meet all inclusion criteria.
- Studies that do not meet the inclusion criteria must be excluded.
- This structured sampling method strengthens the reliability of the literature review based on predefined rules.
- The research team may refine criteria during the review process.
- However, changes must be documented clearly.
- Transparency ensures that readers understand why certain elements of an article were excluded.
How Inclusion Criteria and Exclusion Criteria Shape Research Studies and Clinical Trial Design
- Appropriate inclusion and exclusion criteria directly shape study design.
- In clinical trials, the primary goal is to prove that an intervention is safe and effective.
- To achieve this, researchers must carefully define the appropriate inclusion criteria.
- Target population is crucial in both systematic reviews and clinical research.
- The target population is defined based on the research question.
- Inclusion criteria may specify a particular illness such as heart failure.
- They may specify chronic conditions, comorbidity limitations, or specific disease stages.
- Exclusion criteria must reduce risk and bias.
- Individuals with unstable medical conditions may be excluded for safety reasons.
- Certain groups, such as pregnant women, may be excluded to protect vulnerable populations.
- Exclusion criteria must ensure ethical conduct of the study.
- Criteria influence recruitment and sampling strategies.
- Recruitment materials must clearly specify who is eligible.
- Screening procedures confirm whether individuals meet the inclusion criteria.
- Only eligible participants proceed to enrollment.
- Criteria affect followup and data collection.
- Participants must have the ability to attend study visits and follow-up study assessments.
- Clear eligibility ensures reliable followup and complete data.
- This supports accurate study results.
- In systematic reviews, inclusion and exclusion criteria shape which articles are used to collect data.
- They determine which study design types are included.
- They ensure that selected articles are directly related to the intervention and research question.
- They prevent inclusion of irrelevant or low-quality studies.
- Strong eligibility criteria improve external validity.
- When participants or studies reflect the real-world target population, results are more generalizable.
- However, overly strict exclusion may limit generalizability.
- Researchers must think about criteria carefully to balance internal control and external validity.
- Criteria help ensure the success of the study.
- Clear eligibility supports reliable data collection.
- It strengthens confidence in the results of the study.
- It enhances the overall credibility of clinical research conducted by institutions such as a school of medicine.
- Ultimately, inclusion and exclusion criteria are foundational to any systematic review or clinical trial.
- They are used to select articles.
- They guide screening and recruitment.
- They ensure that study participants are appropriate.
- They protect ethical standards through informed consent and safe conduct.
- They make the findings meaningful, reliable, and generalizable.
Why Are Inclusion Criteria and Exclusion Criteria Important?
Why Exclusion Criteria Important for Study Integrity and Bias Control
- Inclusion criteria and exclusion criteria are essential for protecting the integrity of a study.
- They clearly specify who is eligible and who must be excluded before data collection begins.
- Without appropriate inclusion and exclusion criteria, a research study may include participants who do not align with the research question.
- This misalignment can weaken the study results and threaten the overall success of the study.
- Exclusion criteria are defined to reduce bias.
- Bias can occur when certain characteristics distort the results of the study.
- For example, including participants with multiple chronic conditions when studying a single illness may confound outcomes.
- Exclusion criteria must remove individuals whose additional medical conditions or comorbidity could interfere with accurate measurement of the intervention.
- Bias control is especially important in clinical research and clinical trials.
- The purpose of many clinical trials is to prove that an intervention is safe and effective.
- If exclusion criteria are not carefully applied, the intervention’s effect may be overestimated or underestimated.
- This can lead to incorrect conclusions that affect future clinical research.
- Exclusion criteria help maintain internal validity.
- Internal validity refers to whether the results of the study are truly caused by the intervention and not by other factors.
- By excluding participants with unstable illness, previous treatment history that may interfere, or inability to comply with follow-up, researchers protect the integrity of the findings.
- Structured screening ensures integrity.
- During recruitment and screening, the research team may review inclusion and exclusion criteria with each potential participant.
- Only those who meet the inclusion criteria and do not violate exclusion criteria are allowed to participate in the study.
- This structured conduct ensures consistent sampling and fair evaluation.
- Ethical integrity is also supported by exclusion criteria.
- Some individuals may be excluded for safety reasons, such as pregnant women or individuals with high-risk medical conditions.
- Exclusion criteria must protect vulnerable groups while ensuring informed consent and ethical participation.
- In systematic reviews, exclusion criteria are equally important.
- Researchers must select articles that match predefined eligibility criteria.
- Studies with inappropriate study design, irrelevant intervention, or unclear data may be excluded.
- This prevents inclusion of low-quality evidence in a literature review based on a focused research question.
Need help with a Systematic Literature Review?
Get expert support from Best Dissertation Writers for systematic review writing services.
The Impact of Eligibility Criteria on External Validity
- Eligibility criteria directly influence external validity.
- External validity refers to whether the results are generalizable to the broader target population.
- If the target population is not clearly defined, the results may not apply outside the study sample.
- The target population is crucial when establishing inclusion and exclusion criteria.
- Inclusion criteria may specify demographic characteristics such as age range or gender.
- They may define type and stage of disease, such as heart failure in early versus advanced stages.
- These decisions determine whether participants are representative of the target population.
- Overly restrictive exclusion criteria can limit generalizability.
- If too many individuals are excluded due to comorbidity or previous treatment history, the study participants may not reflect real-world patients.
- As a result, the study results may not be applicable in everyday clinical settings.
- Balanced eligibility criteria strengthen generalizable findings.
- When participants are representative of the target population, the results of the study are more meaningful.
- Clinical research that includes diverse demographic and medical characteristics enhances confidence in broader application.
- In systematic reviews, eligibility criteria determine which studies are included in a literature review.
- If researchers only include highly controlled clinical trials, findings may not represent real-world practice.
- On the other hand, including poorly designed studies may weaken conclusions.
- Thoughtful criteria help maintain a balance between rigor and generalizability.
- Eligibility criteria also affect sampling and recruitment.
- Recruitment materials must clearly specify who is eligible.
- During screening, researchers confirm that individuals meet the inclusion criteria.
- This process ensures that collected data reflect the intended target population.
- External validity depends on clear reporting.
- Researchers must clearly specify the criteria that will be used.
- Transparent reporting allows readers to judge whether the findings are applicable to their own patient populations or research context.
How Inclusion and Exclusion Criteria Affect Clinical Trial Outcomes
- Inclusion and exclusion criteria directly shape clinical trial outcomes.
- Clinical trials is to prove that an intervention is safe and effective.
- The type of participants included in a study can influence how strong or weak the results appear.
- Participant characteristics influence outcomes.
- If inclusion criteria allow only early-stage illness, results may show stronger improvement.
- If participants with severe illness are included, outcomes may appear less favorable.
- Therefore, defining the appropriate inclusion criteria is essential for accurate interpretation.
- Exclusion criteria influence safety outcomes.
- Individuals with unstable medical conditions may be excluded to reduce risk during study visits and followup.
- Clear exclusion ensures participant safety while preserving study integrity.
- Followup and adherence affect results.
- Inclusion criteria may require ability to attend study visits and complete follow-up study assessments.
- Participants who cannot commit to followup may be excluded to prevent missing data.
- Reliable followup strengthens the quality of collected data and improves study results.
- Study design and outcome measurement are connected to eligibility.
- The intervention may work differently in populations with different characteristics.
- Previous treatment history may influence response to a new therapy.
- Carefully selected participants help ensure that measured outcomes truly reflect the intervention effect.
- Eligibility criteria affect statistical power and interpretation.
- Including a homogeneous group reduces variability and may make treatment effects easier to detect.
- However, excessive restriction may limit applicability.
- Researchers must think about criteria strategically to balance precision and real-world relevance.
- Ultimately, inclusion and exclusion criteria are crucial to the success of clinical research.
- They protect study integrity.
- They control bias.
- They shape recruitment and sampling.
- They influence external validity.
- They determine how meaningful and generalizable the results of the study will be.
Examples of Inclusion and Exclusion in Research Studies
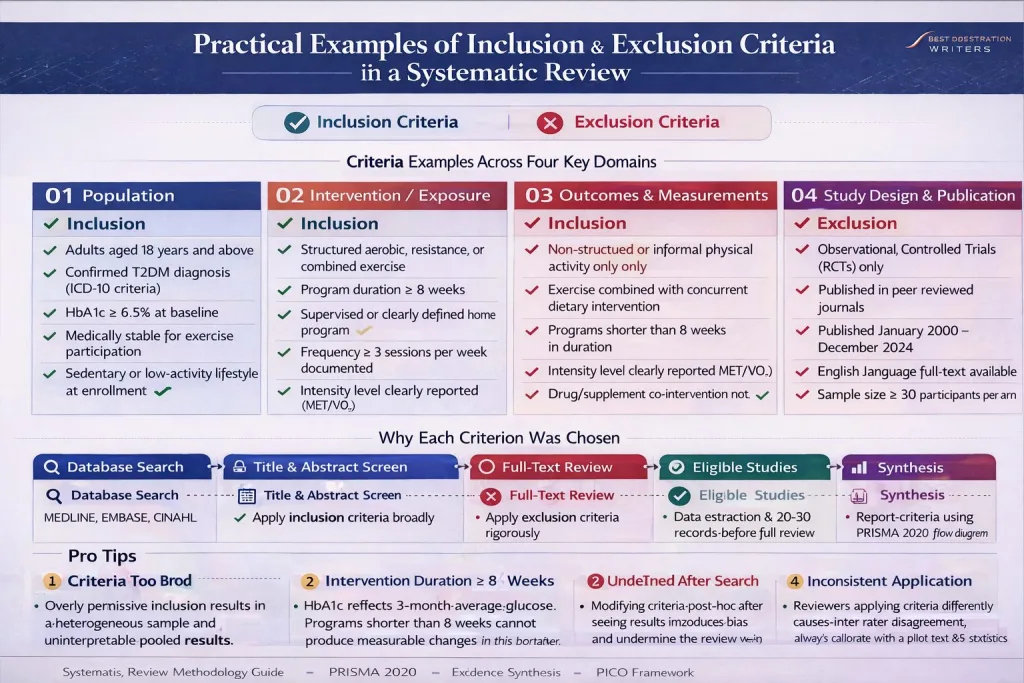
Practical Examples of Inclusion and Exclusion in a Systematic Review
- In a systematic review, inclusion and exclusion criteria are used to select articles that directly answer the research question.
- These criteria are defined before screening begins to ensure consistency and transparency.
- The research team may use databases and libguides at university libraries to retrieve articles for your literature review based on predefined eligibility criteria.
- During screening, researchers review titles, abstracts, and full-text elements of an article to determine whether the study is eligible for inclusion.
- Articles that do not meet the inclusion criteria must be excluded and documented.
Below is a practical example of inclusion in a literature review focusing on heart failure interventions:
| Category | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Adults aged 40–75 diagnosed with heart failure | Pediatric populations or participants without confirmed heart failure |
| Type and Stage | Diagnosed with Stage II or III heart failure | End-stage heart failure or unspecified type and stage |
| Intervention | Drug-based intervention targeting symptom reduction | Surgical procedures unrelated to the intervention |
| Study Design | Randomized controlled trials | Case reports, editorials, narrative reviews |
| Publication Date | Studies published within the last 10 years | Studies older than 10 years |
| Language | English-language articles | Non-English publications |
- In this example, the target population is clearly defined.
- The criteria help ensure that selected articles are directly related to the intervention.
- Exclusion criteria are defined to remove irrelevant or lower-quality evidence.
- This structured sampling strengthens the literature review based on the research question.
Another example may involve reviewing chronic conditions such as diabetes. Inclusion criteria may specify adult populations with a confirmed diagnosis, while exclusion criteria must remove studies involving gestational diabetes or mixed populations without clear subgroup analysis.
Common Eligibility and Exclusion Criterion in Clinical Trial Research
In clinical research and clinical trial design, eligibility criteria determine who can participate in the study. These criteria are essential because clinical trials is to prove that an intervention is safe and effective.
Below is a common example of eligibility criteria in a clinical trial testing a new medication:
| Category | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Age | 18–65 years | Under 18 or over 65 years |
| Diagnosis | Confirmed diagnosis of the illness under study | Absence of confirmed diagnosis |
| Previous Treatment History | No prior exposure to similar intervention | Prior treatment with the same drug |
| Medical Conditions | Stable chronic conditions allowed | Severe comorbidity or unstable illness |
| Pregnancy Status | Non-pregnant individuals | Pregnant women or breastfeeding individuals |
| Ability to Participate | Ability to attend study visits and followup | Inability to comply with follow-up study schedule |
| Consent | Provided informed consent | Refusal or inability to provide informed consent |
- Inclusion criteria may define demographic characteristics, type and stage of disease, and ability to participate in the study.
- Exclusion criteria must prioritize safety and protect vulnerable groups.
- Exclusion criteria with each potential participant are reviewed during screening before recruitment is finalized.
For example, in a clinical trial involving a cardiovascular intervention:
- Participants must meet the inclusion criteria by having confirmed heart failure at a specific stage.
- Individuals with severe renal failure or uncontrolled hypertension may be excluded due to increased risk.
- This approach ensures ethical conduct and protects study integrity.
Eligibility criteria also affect study visits, followup, and data collection. Participants must have the ability to attend appointments and complete questionnaires. Without this, missing data could threaten the success of the study and weaken study results.
In many school of medicine research settings, establishing inclusion and exclusion criteria is a formal process reviewed by ethics committees. The research team may revise criteria if recruitment challenges arise, but changes must be documented carefully.
Need help with a Systematic Literature Review?
Get expert support from Best Dissertation Writers for systematic review writing services.
How to Develop Clear Inclusion Criteria and Exclusion Criteria for Strong External Validity
Developing appropriate inclusion and exclusion criteria requires careful planning. The goal is to balance internal validity with external validity so that results of the study are generalizable.
- Start with the research question.
- Criteria must be based on your research question.
- The target population is crucial when defining eligibility.
- Researchers should think about criteria that align with the intervention and expected outcomes.
- Define the appropriate inclusion criteria.
- Specify demographic factors such as age and sex.
- Define the illness clearly, including type and stage.
- Clarify previous treatment history requirements.
- Ensure participants are representative of the target population.
- Clearly state exclusion criteria.
- Exclusion criteria are defined to remove risks and confounding variables.
- Identify medical conditions or comorbidity that may interfere with results.
- Specify inability to comply with study visits or followup as exclusion factors.
- Ensure feasibility and recruitment practicality.
- Recruitment materials must clearly specify eligibility.
- Screening procedures should be straightforward and reproducible.
- Overly strict exclusion criteria may reduce recruitment success.
- Promote external validity.
- Avoid excluding large segments of the real-world population without strong justification.
- Inclusion criteria may allow stable chronic conditions if relevant to the broader target population.
- When participants are representative, findings are more generalizable.
- Maintain transparency in reporting.
- Researchers must specify all criteria that will be used.
- In systematic reviews, authors must explain why certain articles were excluded.
- In clinical trials, eligibility criteria should be published in trial protocols.
- Review and refine criteria carefully.
- The research team may reassess eligibility during early recruitment phases.
- However, adjustments must not introduce bias.
- Clear documentation ensures credibility.
Strong inclusion and exclusion criteria are crucial to the success of both systematic reviews and clinical research. They help select articles appropriately, protect participant safety, support accurate sampling, guide data collection, and ensure that results of the study are both reliable and generalizable.
